VACCINES
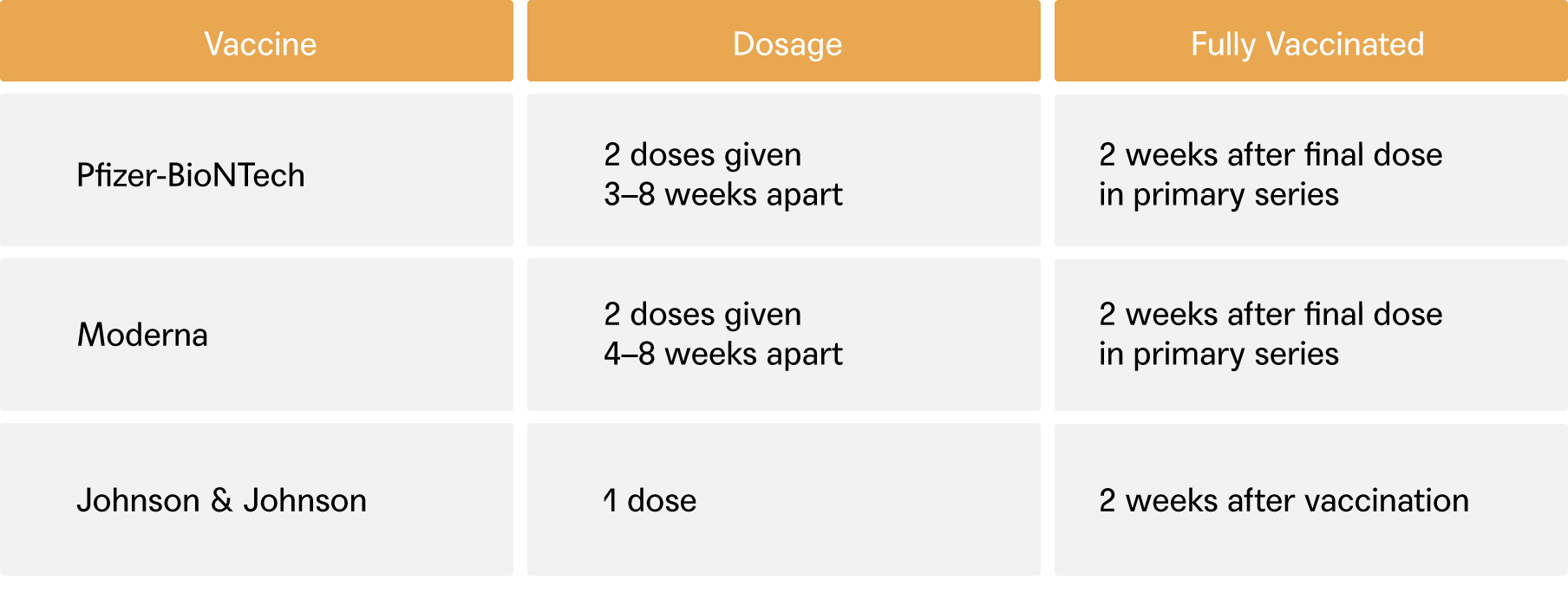
Here are some questions we’ve been getting from our members. These answers may help you, but the best person to answer your vaccine questions is still your physician. If you have questions, just start a conversation or send a message today.
I haven’t been boosted, should I do that now or wait until a newer version of the vaccine becomes available?
Get boosted now. The currently available vaccines are still great at reducing hospitalization and death from current strains of SARS-CoV-2.
Now that vaccines are readily available, is there a benefit to getting all three?
While you can mix and match, there doesn’t seem to be a significant benefit to getting all three vaccines.
I had my first two shots but tested positive for COVID-19 before getting my booster. Should I get my booster and, if so, when?
The research is mixed on this, and there are many variables to consider. In general, any time you are exposed to the virus or a vaccine, your immunity is boosted as a result. That said, the most consistent way to ensure immunity is through vaccination. If you currently have COVID-19, you can get your booster shot as soon as your symptoms go away. Your best bet is to talk this over with your physician to figure out the right steps and timing for you.
I had an anaphylactic reaction to bee stings in the past, should I get vaccinated?
If you’ve had an anaphylactic reaction to anything in the past (bee stings, nuts, medication, etc.), you can still get the vaccine, you will just be monitored for a longer period of time afterwards. Make sure you talk to your physician about any concerns before getting vaccinated.
I’m vaccine hesitant and have reservations about mRNA technology. I heard there is a new vaccine coming called Novovax that is more similar to a traditional vaccine. Should I wait to get that one or get an existing vaccine?
Vaccination is an important part of prevention. If you feel more comfortable not using an mRNA vaccine, you can get the Johnson & Johnson vaccine, or wait until a new vaccine is available. While you’re waiting, it is important to practice other preventive measures like masking, social distancing, and good hand hygiene. You can read more on the CDC’s Prevent Getting Sick page, but always feel free to discuss with your physician.
Should my 5-11 year olds get vaccinated?
In general, children are less frequently infected with COVID-19. When infected, they tend to have less severe symptoms and complications than adults. However, it’s important to understand that children can still get severely ill and die from COVID-19. Also, some children do suffer from the effects of “long COVID-19.” Because of these risks, the American Academy of Pediatrics recommends that all children five and older get vaccinated if they don’t have any contraindications to vaccination.
My teen is an athlete and we’ve heard about the risk of myocarditis with mRNA vaccines. Should I be concerned?
We recommend vaccination because the COVID-19 infection itself can also cause myocarditis. In fact, the risk of myocarditis is greater if you get a COVID-19 infection than if you get the vaccine.
We know you probably have more questions. Start a message with your provider today!
August 24, 2021
The Food and Drug Administration (FDA) announced the full approval of the 2-dose Pfizer-BioNTech COVID-19 vaccine in individuals 16 and older. From the FDA:
The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. The vaccine also continues to be available under emergency use authorization (EUA), including for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
The FDA’s approval of this vaccine is a milestone as we continue to battle the COVID-19 pandemic…While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated.
A statement regarding third booster doses of the Pfizer and Moderna vaccines for the general public was released last week by the U.S. Department of Health and Human Services (HHS). The current plan is to administer a 3rd booster dose of the mRNA COVID-19 vaccines (Pfizer and Moderna) 8 months after an individual’s 2nd dose. This would begin September 20th, subject to the FDA’s independent review of the safety and effectiveness of a 3rd dose of these vaccines as well as the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) recommendation of a booster dose following a thorough review of the evidence. Please see here for additional information on booster doses.
August 18, 2021
While the department of Health and Human Services announced a plan that later this fall booster shots should be obtained by all those already fully vaccinated, the Centers for Disease Control and Prevention (CDC) currently recommends additional doses or booster shots for the following populations:
- Been receiving active cancer treatment for tumors and cancers of the blood
- Received an organ transplant and are taking medications to suppress the immune system
- Received a stem cell transplant within the past 2 years or are taking medications to suppress the immune system
- Moderate or severe primary immunodeficiency (such as DiGeorge syndrome, Wiskott-Aldrich syndrome)
- Advanced or untreated HIV infection
- Active treatment with high-dose steroids or other drugs that may suppress the immune system.
If you feel you are at increased risk for complications from COVID-19 infection, contact your healthcare provider for additional information and advise on receiving additional booster shots.
April 13, 2021
**Official CDC and FDA joint statement:**
https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine
On April 13th, the CDC and FDA released a joint statement regarding a rare but potentially serious type of blood clot that has occurred in six of the more than 6.8 million people who have received the J&J COVID-19 vaccine. Based on this finding, experts are recommending a pause on administration of the vaccine out of an abundance of caution while additional information is gathered. The CDC and FDA statement says that any adverse events in J&J vaccine recipients appear to be extremely rare. Recipients of the vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their healthcare provider.
As a recipient of the J&J vaccine, no special action needs to be taken. Although the likelihood of this type of blood clot is extremely low, it’s important to report the side effects listed above to your doctor if they appear within three weeks of vaccination.
We hold your safety as a top priority and are monitoring closely for the latest updates. Please see below for answers to some important questions about this developing situation and don’t hesitate to contact us with any additional inquiries.
**Is this out of an abundance of caution?**
Yes. The CDC and FDA are being extremely cautious. Although this type of blood clot is rare, it may be potentially serious. It’s important to determine whether these occurrences are truly linked to the vaccine or not.
**Is this a concern for the Pfizer and Moderna vaccines?**
This safety concern was not noted in clinical trials and has not been associated with either of these vaccines at this time.
**Do I need to take aspirin or get another brand of vaccine?**
It is not recommended that you take aspirin or that you be vaccinated with another vaccine.
January 15, 2021
With the recent FDA authorization of two COVID-19 vaccines, we have been getting questions about if and when we will be offering immunizations to our members. While we are not able to offer the vaccine at Crossover Health centers or on-campus locations at this time, here are some points to keep in mind as we anticipate the ability to do so:
- Vaccine supply is still very limited. There are currently two vaccines authorized for emergency use. The federal government is directing distribution to each state—from there, individual states and local health departments are coordinating distribution.
- Each state agency is following their own guidelines and prioritization of which groups can be vaccinated. For most states, the vaccine is only currently available to “Phase 1” individuals, identified as frontline healthcare workers and some residents of long-term care facilities. There are many local variations on the “Phases” which may be set by individual states and/or counties.
- In locations where primary care providers are being offered the vaccine, our care teams are starting to get immunized.
- In each state where we have a health center, Crossover is completing the required registrations to be able to give the COVID-19 vaccine. Once registered, Crossover will need to wait until local health agencies provide vaccine supply to each health center.
- As soon as the vaccine becomes available to Crossover, we will begin to offer them to our members. We anticipate this will be sometime in the spring of 2021 based on current distribution rates.
- As always, our Care Navigators can also assist with finding vaccine dispensing locations for each Phase of recipients.
With two vaccines already authorized by the FDA, the outlook for an end to the pandemic looks to be in sight—although it will be a few more months until the general public will have the ability to be immunized. In the meantime, don’t put off the care you need. We have many health services available to support you—online and in person—from primary care to mental health counseling, physical medicine, and many other resources for sick and preventive care.
We look forward to sharing more information on the COVID-19 vaccination when we are able to begin immunizing.
You may also find our blog about vaccines helpful.
